For the reaction 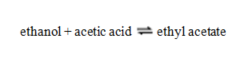 Kc = 0.95. A mixture of the three substances contains 0.45 M ethanol, 0.45 M acetic acid and 1.1 M ethyl acetate. Which statement is true?
Kc = 0.95. A mixture of the three substances contains 0.45 M ethanol, 0.45 M acetic acid and 1.1 M ethyl acetate. Which statement is true?
A) Q < K, so the system will react left to right.
B) Q < K, so the system will react right to left.
C) The mixture is at equilibrium.
D) Q > K, so the system will react left to right.
E) Q > K, so the system will react right to left.
Correct Answer:
Verified
Q24: Consider the reaction Q25: Consider the endothermic reaction Q26: Which of the following is false? Q27: For the reaction Q28: Consider the reaction Q30: If the reaction quotient, Q, is greater Q31: For the reaction Q32: Once the reaction quotient, Q, has been Q33: For the reaction Q34: Consider the reaction Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
A) a![]()
![]()
![]()
![]()
![]()

