Consider the equilibrium system 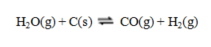 If C is removed, the equilibrium will ____, and if CO is added, the equilibrium will ____.
If C is removed, the equilibrium will ____, and if CO is added, the equilibrium will ____.
A) shift to the left; shift to the left
B) shift to the right; shift to the right
C) shift to the right; shift to the left
D) be unchanged; shift to the left
E) be unchanged; shift to the right
Correct Answer:
Verified
Q16: A chemical equilibrium may involve
A) reactants and
Q17: The equilibrium constant for a particular chemical
Q18: If a catalyst is added to a
Q19: Which of the following is not true
Q20: A chemical reaction reaches equilibrium when
A) both
Q22: To decide whether a reaction mixture is
Q23: An endothermic reaction which results in an
Q24: Consider the reaction Q25: Consider the endothermic reaction Q26: Which of the following is false?![]()
![]()
A) a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents