The decomposition of N2O(g) to nitrogen and oxygen has the rate law 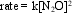 where k = 1.1 × 10-3 M-1 s-1 at 565 C. If the initial concentration of a sample of N2O is 0.50 M, what is the concentration after one hour at 565 C?
where k = 1.1 × 10-3 M-1 s-1 at 565 C. If the initial concentration of a sample of N2O is 0.50 M, what is the concentration after one hour at 565 C?
A) 9.5 × 10-3 M
B) 2.2 × 10-3 M
C) 0.029 M
D) 0.17 M
E) 0.46 M
Correct Answer:
Verified
Q16: For a reaction that is zeroth-order with
Q17: For a new chemical reaction involving several
Q18: Which of the following units may be
Q19: Which of the following factors will affect
Q20: The rate of a chemical reaction may
Q22: In a bimolecular reaction, which term can
Q23: A certain reaction is studied at room
Q24: From the stoichiometry of the reaction
Q25: The Arrhenius equation is Q26: The half-life for the first-order conversion of
A) ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents