What volume (in L) of oxygen at 298K and 1.50 atm is required for the complete combustion of 25.0 g of hexane? The value of R = 0.0821 L atm mol-1 K-1. 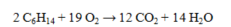
A) 4.73 L
B) 0.397 L
C) 34.2 L
D) 45.0 L
E) 407 L
Correct Answer:
Verified
Q26: Calcium carbonate decomposes upon heating to form
Q27: The following is the reaction that occurs
Q28: What conditions are referred to as standard
Q29: How are the pressure and the absolute
Q30: A 125.0-mL contains a gas at 27°C
Q32: Suppose that at STP a gas occupies
Q33: What is the density of oxygen gas
Q34: Derive the gas constant (in L torr
Q35: What is the molar mass of an
Q36: Which is a summary of Avogadro's Law?
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents