Solid potassium chlorate can be decomposed to produce potassium chloride and oxygen according to the following equation. 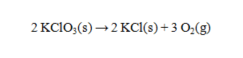 How many grams of KClO3 are needed to produce 752 mL of oxygen at 23°C and 0.995 atm, if the oxygen is collected over water? The vapor pressure of water at this temperature is 21.07 torr.
How many grams of KClO3 are needed to produce 752 mL of oxygen at 23°C and 0.995 atm, if the oxygen is collected over water? The vapor pressure of water at this temperature is 21.07 torr.
A) 0.0308 g
B) 2.43 g
C) 2.52 g
D) 3.66 g
E) 3.77 g
Correct Answer:
Verified
Q47: Hydrochloric acid reacts with magnesium to produce
Q48: How many liters does 3.336 g of
Q49: For a gas mixture
A) the partial pressures
Q50: Suppose that 1.00 gram of each of
Q51: Which is not a greenhouse gas?
A) carbon
Q53: If 7.63 g of an unknown gas
Q54: What is the density (in g/L) of
Q55: What is the pressure of 2.50 mol
Q56: A 4.35-g sample of gas has a
Q57: Which description of ideal gases and real
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents