When atoms of aluminum-27 are bombarded with alpha particles, a neutron and an element are produced.Which particular isotope of this element is formed? 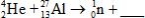
A) 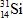
B) 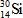
C) 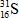
D) 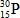
E) 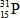
Correct Answer:
Verified
Q1: Uranium-235 decays by alpha emission. What isotope
Q2: When atoms of beryllium−9 are bombarded with
Q2: What is the name for spontaneous emission
Q4: Which is an incorrect representation of the
Q10: What is the missing symbol in this
Q11: What other particle is emitted when a
Q12: Beta particles are identical to
A) protons.
B) helium
Q14: In the equation below, what particle or
Q19: In the equation below, what particle or
Q21: What fraction of radioactive atoms remains in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents