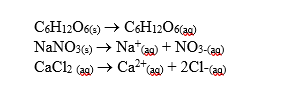
-One mole of particles lowers the freezing point of 1,000 g of water by 1.76ºC.What is the freezing point of a solution containing one mole of NaNO3 in 1,000 g of water?
A) 30.24ºC
B) 28.48ºC
C) -1.76ºC
D) -3.52ºC
Correct Answer:
Verified
Q13: Water solutions of ionic substances that conduct
Q14: What is the most likely temperature of
Q15: Hydrogen bonding in water accounts for
A)water's higher
Q16: A strong acid is a concentrated solution
Q17: The rule "like dissolves like" explains why
Q19: Cooks add a pinch of salt to
Q20: Polar compounds such as alcohol would be
Q21: What do solutions of acids, bases, and
Q22: A hydrogen bond is
A)what holds the atoms
Q23: Icebergs
A)have about the same density as sea
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents