There are two stable isotopes of chlorine: chlorine-35, with a mass of 34.968853 amu; and chlorine-37, with a mass of 36.965903. Given that the average atomic mass of a chlorine atom is 35.45 amu, which of the following statements is true?
A) Chlorine contains almost exclusively of 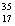 Cl, with very little
Cl, with very little 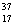 Cl.
Cl.
B) Chlorine contains more 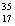 Cl than
Cl than 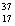 Cl.
Cl.
C) Chlorine contains roughly equal amounts of 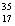 Cl and
Cl and 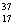 Cl.
Cl.
D) Chlorine contains more 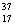 Cl than
Cl than 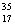 Cl.
Cl.
E) Chlorine contains almost exclusively of 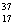 Cl, with very little
Cl, with very little 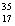 Cl.
Cl.
Correct Answer:
Verified
Q4: One nanogram does not seem like a
Q9: The mass of 1.63 * 1021 silicon
Q11: One mole of iron
A) is heavier than
Q11: An atom of helium has a mass
Q12: What is the average mass, in grams,
Q15: An atom of bromine has a mass
Q20: What is the average mass, in grams,
Q28: What is the molar mass of nicotine,
Q31: A silver wire has a diameter of
Q35: How many atoms are in 4.39 g
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents