Solid sodium hydrogen carbonate (also known as sodium bicarbonate) can be decomposed to form solid sodium carbonate, gaseous carbon dioxide, and water vapor. When the balanced chemical reaction for this process is written such that the coefficient of water is 1, what is the coefficient of carbon dioxide?
A) 0
B) 1
C) 2
D) 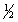
E) cannot be determined
Correct Answer:
Verified
Q102: Calculate the molecular mass, in g/mol, of
Q105: Liquid hexane, C6H14, burns in oxygen gas
Q106: Which of the following statements are true
Q107: Liquid heptane, C7H16, burns in oxygen gas
Q109: Calculate the molecular mass, in g/mol, of
Q112: A method for producing pure copper metal
Q117: The Hall process for the production of
Q136: How many moles of iron are present
Q148: How many ICl3 molecules are present in
Q158: Calculate the molecular mass, in g/mol, of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents