What is the pressure of the sample of gas trapped in the open-tube mercury manometer shown below if atmospheric pressure is 736 mmHg and h = 9.2 cm? 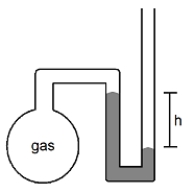
A) 92 mmHg
B) 644 mmHg
C) 736 mmHg
D) 828 mmHg
Correct Answer:
Verified
Q1: At what temperature will a fixed amount
Q2: The pressure of a gas sample was
Q4: The gas pressure in an aerosol can
Q5: What will happen to the height (h)of
Q5: If the pressure on a gas sample
Q6: The temperature of an ideal gas in
Q9: A sample of nitrogen gas has a
Q11: At what temperature will a fixed mass
Q15: A sample of a gas occupies 1.40
Q16: A pressure that will support a column
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents