What is the pressure of the sample of gas trapped in the open-tube mercury manometer shown below if atmospheric pressure is 742 mmHg and h = 16.7 cm? 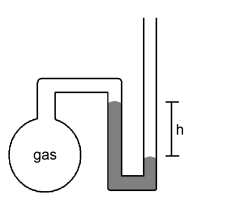
Correct Answer:
Verified
Q46: A gaseous compound is 30.4% nitrogen and
Q48: Calculate the volume of H2(g)at 273 K
Q62: What is the pressure of the gas
Q63: What is the pressure of the sample
Q68: Determine the pressure of the gas trapped
Q70: For a substance that remains a gas
Q87: What is the definition of a "gas"?
Q90: A spacecraft is filled with 0.500 atm
Q93: Samples of the following volatile liquids are
Q96: A spacecraft is filled with 0.500 atm
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents