At 25°C, the following heats of reaction are known: 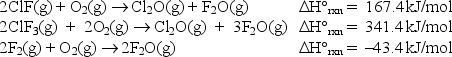 At the same temperature, use Hess's law to calculate H°rxn for the reaction: ClF(g) + F2(g) ClF3(g)
At the same temperature, use Hess's law to calculate H°rxn for the reaction: ClF(g) + F2(g) ClF3(g)
A) -217.5 kJ/mol
B) -130.2 kJ/mol
C) 217.5 kJ/mol
D) -108.7 kJ/mol
E) 465.4 kJ/mol
Correct Answer:
Verified
Q61: A gas is compressed in a cylinder
Q72: For which of these reactions will
Q73: The bond enthalpy of the Br-Cl
Q74: The heat of solution of ammonium nitrate
Q92: The enthalpy of combustion of acetylene
Q93: A 0.3423 g sample of pentane, C5H12,
Q95: Define specific heat.
Q96: How many grams of ethylene (C2H4)would
Q97: A 0.1946 g piece of magnesium metal
Q98: Calculate the enthalpy of reaction for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents