Given the following H° values,
H2(g)+ 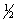 O2(g) H2O(l) H°f = -285.8 kJ/mol
O2(g) H2O(l) H°f = -285.8 kJ/mol
H2O2(l) H2(g)+ O2(g) H°rxn = 187.6 kJ/mol
calculate H°rxn for the reaction H2O2(l) H2O(l)+ 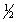 O2(g),
O2(g),
Correct Answer:
Verified
Q63: Ozone (O3)in the atmosphere can be
Q65: Which of the following processes always results
Q66: The heat of solution of ammonium chloride
Q67: A gas is allowed to expand,
Q72: For which of these reactions will
Q73: The bond enthalpy of the Br-Cl
Q74: The heat of solution of ammonium nitrate
Q92: The enthalpy of combustion of acetylene
Q94: A 26.2 g piece of copper metal
Q97: A 0.1946 g piece of magnesium metal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents