A photon is roughly 1800 times more massive than an electron. If a proton and an electron have the same kinetic energy,
A) the wavelength of the photon will be about 1800 times longer than the wavelength of the electron.
B) the wavelength of the photon will be about 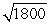 times longer than the wavelength of the electron.
times longer than the wavelength of the electron.
C) the wavelength of the photon will be roughly equal to the wavelength of the electron.
D) the wavelength of the electron will be about 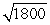 times longer than the wavelength of the photon.
times longer than the wavelength of the photon.
E) the wavelength of the electron will be about 1800 times longer than the wavelength of the photon.
Correct Answer:
Verified
Q5: Calculate the wavelength of the light emitted
Q11: Calculate the frequency of the light emitted
Q12: As the frequency of an electromagnetic wave
Q14: Calculate the frequency of visible light having
Q18: What is the wavelength of radiation that
Q18: What is the energy in joules of
Q27: In an electron microscope, electrons are accelerated
Q31: A single pulse of a laser
Q35: Electrons can be used to probe the
Q42: "No two electrons in an atom can
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents