Calculate the Energy Change for the Reaction K(g)+ I(g) K+(g)+ I - (G)
Given the Following Ionization Energy (IE)and
Calculate the energy change for the reaction K(g) + I(g) K+(g) + I - (g)
Given the following ionization energy (IE) and electron affinity (EA) values. 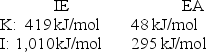
A) -124 kJ/mol
B) -715 kJ/mol
C) 715 kJ/mol
D) 1429 kJ/mol
E) None of these
Correct Answer:
Verified
Q4: Complete this statement: Coulomb's law states
Q13: Which one of the following is most
Q14: Which of the following solids would have
Q15: The Lewis dot symbol for the a
Q16: The Lewis dot symbol for the S
Q18: Which of the following ionic solids would
Q19: Which one of the following is most
Q20: Which one of the following ionic solids
Q25: Which of the following covalent bonds is
Q30: Which of the elements listed below is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents