Multiple Choice
Which of the following Lewis structures is incorrect?
A) 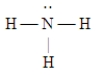
B) 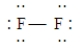
C) 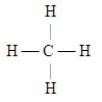
D) 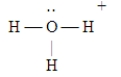
E) 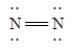
Correct Answer:
Verified
Related Questions
Q42: The electron dot formula for O2 shows
A)
Q48: What is the formal charge on the
Q49: Classify the Ca - Cl bond in
Q54: The number of lone electron pairs in
Q63: The number of resonance structures for the
Q64: Assuming the octet rule is obeyed, how
Q68: The formal charge on the bromine atom
Q71: Which of the following is a useful
Q77: The total number of lone pairs in
Q79: Assuming the octet rule is obeyed, how
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents