Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below? 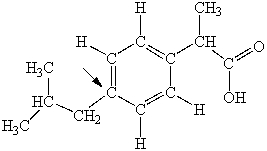
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer:
Verified
Q41: Which one of the following molecules is
Q48: Which one of the following molecules is
Q53: Ibuprofen is used as an analgesic for
Q54: N,N-diethyl-m-tolumide (DEET)is the active ingredient in many
Q55: Complete this sentence: The PCl5 molecule has
A)nonpolar
Q60: According to the VSEPR theory, the actual
Q60: N,N-diethyl-m-tolumide (DEET)is the active ingredient in many
Q66: What is the hybridization of the As
Q80: What is the hybridization of As in
Q82: Consider the species Cl2+, Cl2, and Cl2-.Which
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents