Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. 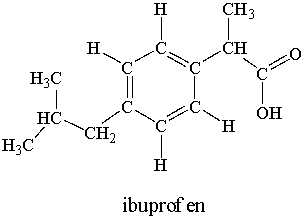 How many sigma bonds and pi bonds are contained in an ibuprofen molecule?
How many sigma bonds and pi bonds are contained in an ibuprofen molecule?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q84: In which of the following would the
Q98: N,N-diethyl-m-tolumide (DEET)is the active ingredient in many
Q105: Which of the following is not true
Q106: Which of the following correctly lists species
Q111: According to VSEPR theory, which of the
Q111: A homonuclear diatomic molecule is a molecule
Q117: Use VSEPR theory to predict the molecular
Q124: Pi bonds are covalent bonds in which
Q130: What bond angles are predicted by VSEPR
Q137: According to the VSEPR theory, all of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents