The systematic name for the compound represented below is 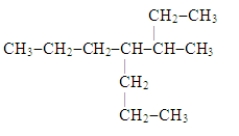
A) 4,5-diethylheptane.
B) 3-propyl-4-ethylhexane.
C) 3-ethyl-4-propylhexane.
D) 3-methyl-4-propylheptane.
E) 2-ethyl-4-propylhexane.
Correct Answer:
Verified
Q1: A particular structural isomer of C6H14 is
Q1: The alkane with six carbon atoms is
Q2: Which of these is the systematic name
Q2: Unsaturated hydrocarbons
A) contain at least one double
Q3: Alkanes have the general formula
A) CnH2n-4.
B) CnH2n-2.
C)
Q3: The octane rating of gasoline refers to
Q3: The two molecules represented below are examples
Q6: The formula CH3CH2CH2CH=CH2 represents
A) an alkane.
B) a
Q7: The compound that has a triple bond
Q9: Cycloalkanes have the general formula
A) CnH2n-4.
B) CnH2n-2.
C)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents