Use the graph of vapor pressure to determine the normal boiling point of O2. 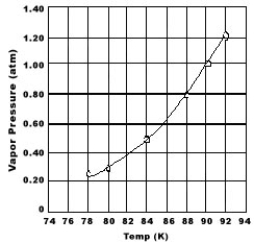
A) 92 K
B) 90 K
C) 88 K
D) 84 K
E) O2 doesn't boil because it is always a gas.
Correct Answer:
Verified
Q42: Potassium bromide, KBr, crystallizes like NaCl in
Q43: The number of nearest neighbors (atoms that
Q45: Use the graph of vapor pressure to
Q48: The zincblende structure of ZnS has the
Q49: Silver metal crystallizes in a face-centered cubic
Q49: Potassium crystallizes in a body-centered cubic lattice.
Q51: The mineral manganosite, manganese(II)oxide, crystallizes in the
Q61: The specific heat of liquid ethanol, C2H5OH(l),
Q62: Acetic acid has a heat of fusion
Q71: Calculate the amount of heat needed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents