Based on the phase diagram shown below, how will the melting point of the substance change if the pressure is increased above 1 atm? 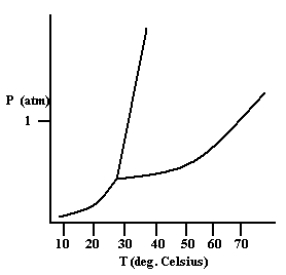
A) The melting point will decrease.
B) The melting point will remain the same.
C) The melting point will increase.
D) The substance will not melt at pressures of 1 atm and above; instead, the solid sublimes to form the gas phase.
Correct Answer:
Verified
Q63: The normal boiling point of methanol (CH3OH)is
Q64: The vapor pressure of ethanol is 400
Q65: Which one of the following elements would
Q67: The molar enthalpy of vaporization of carbon
Q73: Which of the following gases would have
Q78: The molar enthalpy of vaporization of hexane
Q81: Octane is a liquid component of gasoline.Given
Q82: The molar enthalpy of vaporization of boron
Q94: Identify the dominant (strongest)type of intermolecular force
Q113: Indicate all the types of intermolecular forces
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents