What is the slope of an Arrhenius plot for the following reaction? 2NOCl 2NO + Cl2 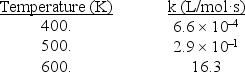
A) 8.18 * 10-2 K
B) 5.06 * 10-2 K
C) -1.22 * 104 K
D) -1.96 * 104 K
E) not enough information to calculate the slope
Correct Answer:
Verified
Q43: For what order reaction does the half-life
Q48: For a second order reaction, the half-life
Q52: If Ea for a certain biological reaction
Q53: For the chemical reaction A
Q58: For the reaction X + Y
Q68: The activation energy for the following first-order
Q72: The activation energy for the reaction
Q72: Given that Ea for a certain biological
Q92: The activation energy of a certain uncatalyzed
Q97: Which of the following statements is false
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents