For the chemical reaction system described by the diagram below, which statement is true? 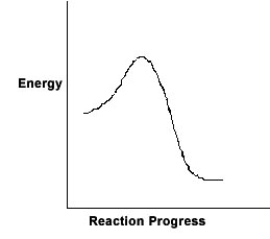
A) The forward reaction is endothermic.
B) The activation energy for the forward reaction is greater than the activation energy for the reverse reaction.
C) At equilibrium, the activation energy for the forward reaction is equal to the activation energy for the reverse reaction.
D) The activation energy for the reverse reaction is greater than the activation energy for the forward reaction.
E) The reverse reaction is exothermic.
Correct Answer:
Verified
Q64: The reaction C4H10
Q65: A reaction mechanism usually is
A)the same as
Q73: The rate law for the reaction
Q76: The following reaction in aqueous solution
Q78: The gas phase reaction of nitrogen
Q83: Complete the following statement: A catalyst
A) increases
Q86: Sucrose, C12H22O11, reacts slowly with water in
Q87: The first-order decomposition of SO2Cl2 to sulfur
Q89: The rate law for the reaction
Q98: For the reaction X2 + Y
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents