With respect to the figure below, which choice correctly identifies all the numbered positions? 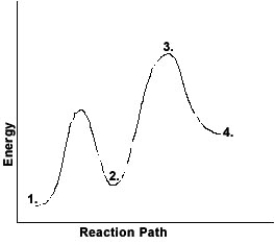

A) 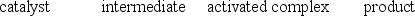
B) 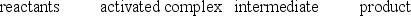
C) 
D) 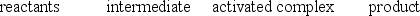
E) 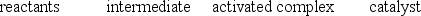
Correct Answer:
Verified
Q64: The reaction C4H10
Q65: An increase in the temperature of the
Q67: For the chemical reaction system described by
Q69: When the concentrations of reactant molecules are
Q73: The activation energy for the reaction
Q73: The rate law for the reaction
Q76: Solids cannot react with gases.
A)1 and 2
B)1
Q83: Complete the following statement: A catalyst
A) increases
Q98: Nitrous oxide (N2O)decomposes at 600°C according
Q98: For the reaction X2 + Y
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents