Consider the chemical reaction 2NH3(g) 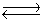 N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Estimate the equilibrium concentration of H2(g).
N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Estimate the equilibrium concentration of H2(g).
Correct Answer:
Verified
Q66: Consider the equilibrium equation C(s)+ H2O(g)+ 2296
Q67: Consider the equilibrium equation C(s)+ H2O(g)+ 2296
Q68: Hydrogen iodide decomposes according to the equation:
2HI(g)
Q69: The data below refer to the following
Q70: Ethanol and acetic acid react to form
Q72: The data below refer to the following
Q73: Consider the reaction N2(g)+ 3H2(g) 
Q74: Consider the reaction N2(g)+ 3H2(g) 
Q75: Consider the equilibrium equation C(s)+ H2O(g)+ 2296
Q85: What conditions are used in the Haber
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents