Consider the chemical reaction 2NH3(g) 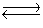 N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Estimate the equilibrium concentration of N2(g).
N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Estimate the equilibrium concentration of N2(g).
Correct Answer:
Verified
Q60: For the common allotropes of carbon
Q61: The data below refer to the following
Q62: Hydrogen iodide decomposes according to the equation:
2HI(g)
Q64: Calcium carbonate decomposes at high temperatures to
Q66: Consider the equilibrium equation C(s)+ H2O(g)+ 2296
Q67: Consider the equilibrium equation C(s)+ H2O(g)+ 2296
Q68: Hydrogen iodide decomposes according to the equation:
2HI(g)
Q69: The data below refer to the following
Q70: Ethanol and acetic acid react to form
Q74: The dissociation of solid silver chloride in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents