The equilibrium constant for the chemical equation
2NO(g)+ O2(g) 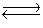 2NO2(g)
2NO2(g)
is two times the equilibrium constant for the chemical equation
NO(g)+ 1/2O2(g) 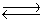 NO2(g).
NO2(g).
Correct Answer:
Verified
Q78: Consider the reaction N2(g)+ 3H2(g) 
Q79: Consider the equilibrium equation C(s)+ H2O(g)+ 2296
Q80: Calcium carbonate decomposes at high temperatures to
Q81: For the reaction H2(g)+ I2(g) 
Q82: The equilibrium between carbon dioxide gas and
Q84: The equilibrium constant expression for the reaction
CuO(s)+
Q85: Equilibrium constants are known for the
Q86: Kc for the reaction CO2(g)+ H2(g)
Q87: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q88: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents