Calculate G° for the combustion of ethanol vapor, C2H5OH(g) , at 750°C in oxygen to form carbon dioxide and water vapor. The following data is valid at 25°C: 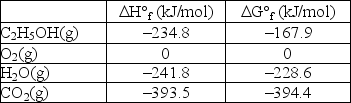
A) -1407 kJ/mol
B) -2151 kJ/mol
C) -1307 kJ/mol
D) -4486 kJ/mol
E) -1377 kJ/mol
Correct Answer:
Verified
Q46: Which species will have the greatest absolute
Q55: Find the temperature at which Kp
Q56: The solubility product constant at 25°C
Q57: For the reaction CuS(s)+ H2(g)
Q58: A sample of solid naphthalene is introduced
Q61: For the reaction 3H2(g)+ N2(g)
Q64: Predict the sign of
Q71: Assuming
Q78: Predict the sign of
Q82: Is the reaction SiO2(s)+ Pb(s)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents