Assuming a coordination complex is formed with Fe2+ and 1,10-phenanthroline (shown below) , which of the following statements is true? 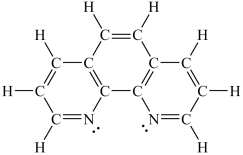
A) If two 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 2.
B) If two 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 6.
C) If three 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 3.
D) If three 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 6.
E) If four 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 4.
Correct Answer:
Verified
Q1: The electron configuration of a Ti atom
Q6: The electron configuration of a Co3+ ion
Q8: The total number of electrons in the
Q10: How many 3d electrons does a V3+
Q12: In the complex ion [Fe(CN)6]4-, the oxidation
Q15: In K4[Fe(CN)6], how many 3d electrons does
Q16: The total number of electrons in the
Q20: The electron configuration of a Cr3+ ion
Q23: Which response gives the correct coordination number
Q37: In the complex ion [Cr(C2O4)2(H2O)2]-, the oxidation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents