Give (a)the oxidation number of the metal, (b)the number of d electrons, (c)the coordination number, (d)the charge of the complex ion, and (e)the number and type of ligands for the coordination compound shown below. 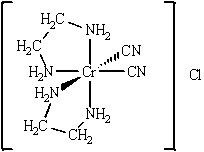
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q65: The systematic name of the coordination compound
Q66: In the following pair of complex ions,
Q78: The correct formula for the dibromobis(oxalato)cobaltate(III)ion is
Q83: Name the complex ion [Co(H2O)4Cl2]+.
Q83: In transition metal complexes, the metal ions
Q84: Predict the number of unpaired electrons in
Q87: The neutral monodentate ligand L forms the
Q87: The oxidation number of Co in [Co(NH3)4Cl2]Cl
Q88: Predict the number of unpaired electrons in
Q92: Name the complex ion [Cr(en)2(H2O)2]2+.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents