How many neutrons and protons (nucleons) does an atom with the symbol 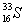 have?
have?
A) 33
B) 16
C) 49
D) None of the above.
Correct Answer:
Verified
Q2: Alpha particles are identical to
A) protons.
B) helium
Q11: What is the nuclear binding energy per
Q12: Beta particles are identical to
A) protons.
B) helium
Q12: Predict the other product of the following
Q14: The isotope with the greatest nuclear binding
Q17: Radium-226 decays by alpha emission. What is
Q18: The only stable isotope of iodine is
Q19: The only stable isotope of aluminum is
Q21: Determine how much energy is released when
Q39: A typical radius of an atomic nucleus
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents