Estimate the age of a bottled wine that has a tritium, 3H, content 60% that of freshly bottled wine. Tritium decays by beta decay and has a half-life of 12.3 yr. 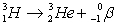
A) 0.029 yr
B) 7.4 yr
C) 9.1 yr
D) 16 yr
E) 35 yr
Correct Answer:
Verified
Q21: What fraction of radioactive atoms remains in
Q23: The 14C activity of some ancient Peruvian
Q34: The only stable isotope of fluorine is
Q36: The heaviest known isotope of hydrogen is
Q41: What would the atom ratio of 206Pb
Q48: Charcoal found under a stone at Stonehenge,
Q53: How old is a bottle of wine
Q58: If 12% of a certain radioisotope decays
Q60: Polonium-208 is an alpha emitter with a
Q70: The carbon-14 activity of some ancient Indian
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents