Ascorbic acid,C6H806,can be represented by the molecular model shown below.If 1.00 mol of ascorbic acid is submitted to combustion analysis,how many moles of CO2 and how many moles of H2O would be formed? 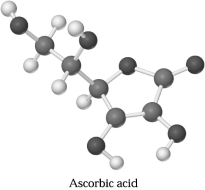
A) 3) 00 mol CO2 and 2.00 mol H2O
B) 6) 00 mol CO2 and 4.00 mol H2O
C) 6) 00 mol CO2 and 8.00 mol H2O
D) 12.0 mol CO2 and 10.0 mol H2O
Correct Answer:
Verified
Q103: Aluminum metal reacts with aqueous iron(II)chloride to
Q126: A hydrocarbon of unknown formula CxHy was
Q129: Glucose,C6H1206,can be represented by the molecular model
Q132: How many moles are there in 3.00
Q133: What mass of carbon dioxide,C O2,contains the
Q134: What mass of phosphorus pentafluoride,PF5,has the same
Q134: What is the molar mass of 1-butene
Q142: How many oxygen atoms are there in
Q147: How many chloride ions are there in
Q148: How many cations are there in 10.0
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents