Multiple Choice
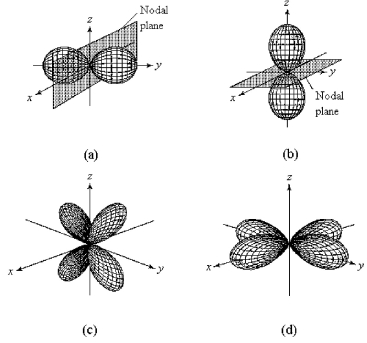
-Which of the above fourth-shell orbitals is a 4dx2-y2 orbital?
A) orbital (a)
B) orbital (b)
C) orbital (c)
D) orbital (d)
Correct Answer:
Verified
Related Questions
Q57: If the quantum number ms had possible
Q58: Which statement is false?
A)For any atom,the 4s
Q59: The first vibrational level for NaH lies
Q60: The symbol [Kr] represents
A)4s24p6.
B)1s22s22p63s23p64s24p6.
C)1s22s22p63s23p63d104s24p6.
D)1s22s22p63s23p63d104s24p64d10.
Q61: For the fourth-shell orbital shown below,what are
Q63: For the fourth-shell orbital shown below,what are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents