What is the ground-state valence-shell electron configuration of the group of elements indicated by the shaded portion of the periodic table? 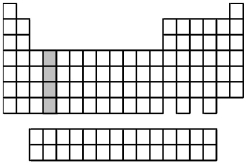
A) ns2
B) ns2np2
C) ns2(n-1) d2
D) ns2(n-2) f2
Correct Answer:
Verified
Q45: Two electromagnetic waves are represented below.
Q50: Molybdenum has an anomalous electron configuration.Write the
Q68: Q76: How many unpaired electrons are in an Q77: Which atom in each group (I and Q78: A neutral sulfur atom has how many Q82: Which period of elements,indicated by letter on Q84: Which groups of elements,indicated by letter on Q86: For the fourth-shell orbital shown below,what are Q96: ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents