Calculate the lattice energy for MgO(s) using a Born-Haber cycle and the following information: 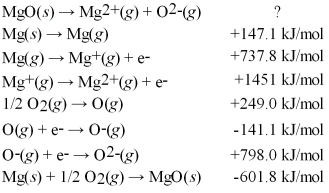
A) +1842 kJ/mol
B) +2444 kJ/mol
C) +3844 kJ/mol
D) +4108 kJ/mol
Correct Answer:
Verified
Q5: Which is not a chemical reaction of
Q26: Which is not generally considered to be
Q27: In the reaction of sodium metal with
Q28: The alkali metals K,Rb,and Cs are commercially
Q43: Which chemical process is associated with the
Q54: Which species does not have an octet
Q55: Which of the following ionic compounds would
Q60: Calculate the lattice energy for MgCl2(s)using a
Q61: Which alkaline earth metal reacts the most
Q119: How many valence shell electrons does an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents