Calculate the electron affinity for the formation of the hydride ion from the following information: 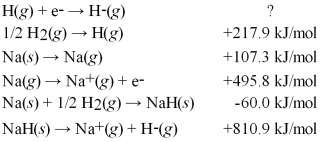
A) -50.1 kJ/mol
B) -70.1 kJ/mol
C) -816 kJ/mol
D) -1632 kJ/mol
Correct Answer:
Verified
Q27: In the reaction of sodium metal with
Q28: What is the general trend in ionization
Q32: Which of these elements has the most
Q33: An element that has the valence electron
Q43: Which chemical process is associated with the
Q49: Calculate the energy change in kJ/mol for
Q51: How many electrons does magnesium lose and
Q53: The octet rule is most likely to
Q54: Which species does not have an octet
Q55: Which of the following ionic compounds would
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents