Use the given average bond dissociation energies,D,to estimate ΔH for the reaction of methane,CH4(g) ,with fluorine according to the equation:
CH4(g) + 2 F2(g) → CF4(g) + 2 H2(g) 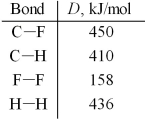
A) -716 kJ
B) -318 kJ
C) +318 kJ
D) +716 kJ
Correct Answer:
Verified
Q46: Ethyl alcohol is produced by the fermentation
Q47: Determine the sign of ΔS° for each
Q53: Which of CH4(g),C2H2(g),and CH3OH(l)provides the most energy
Q54: Which thermodynamic function is most related to
Q61: Reactant R reacts with reactant S in
Q69: If an endothermic reaction is spontaneous at
Q73: Determine the sign of ΔS° for each
Q75: For the reaction,NH3(g)→ N(g)+ 3 H(g),one would
Q79: At a given temperature and pressure,which of
Q82: Imagine a reaction that results in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents