Calculate the enthalpy of combustion per mole for C6H12O6.Assume that the combustion products are CO2(g) and H2O(l) . 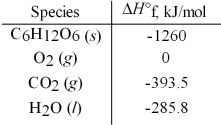
A) -5336 kJ/mol
B) -2816 kJ/mol
C) -1939 kJ/mol
D) 580.7 kJ/mol
Correct Answer:
Verified
Q45: For the reaction 2CH4 (g)+ 3 Cl2
Q47: Determine the sign of ΔS° for each
Q50: Which of the following can be interpreted
Q53: Which of CH4(g),C2H2(g),and CH3OH(l)provides the most energy
Q54: Which thermodynamic function is most related to
Q66: Acetylene torches utilize the following reaction:
2 C2H2(g)+
Q67: One method for making ethanol,C2H5OH,involves the gas-phase
Q68: Given that ΔH°= -311 kJ for the
Q71: For the expansion of an ideal gas
Q75: For the reaction,NH3(g)→ N(g)+ 3 H(g),one would
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents