Which substance in each of the following pairs is expected to have the larger dispersion forces? 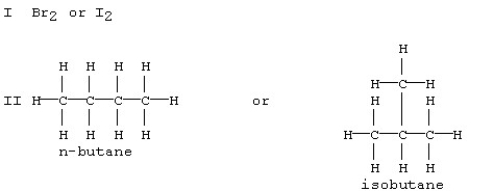
A) Br2 in set I and n-butane in set II
B) Br2 in set I and isobutane in set II
C) I2 in set I and n-butane in set II
D) I2 in set I and isobutane in set II
Correct Answer:
Verified
Q4: When a narrow diameter glass tube is
Q10: Which covalent bond is the most polar?
A)N-F
B)C-F
C)Cl-F
D)F-F
Q11: Which has a dipole moment?
A)CO2
B)CO32-
C)SO2
D)SO42-
Q12: Which of the following compounds exhibits hydrogen
Q15: In liquid methanol,CH3OH,which intermolecular forces are present?
A)Dispersion,hydrogen
Q16: Which compound,shown with its dipole moment,is expected
Q17: Which has the smallest dipole-dipole forces?
A)CH3F
B)HCl
C)N2
D)CO
Q18: Which of the following compounds exhibits only
Q29: The dipole moment of BrF is 1.29
Q142: Which of the following should have the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents