Multiple Choice
The plots below represent vapor pressure vs.temperature curves for diethyl ether,ethanol,mercury,and water,not necessarily in that order. 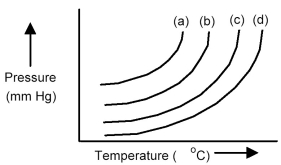
-Based on the relative strengths of the intermolecular forces of attraction of each substance,which is the most likely vapor pressure vs.temperature curve for diethyl ether?
A) curve (a)
B) curve (b)
C) curve (c)
D) curve (d)
Correct Answer:
Verified
Related Questions
Q55: KBr crystallizes in a cubic unit cell

