The plots below represent vapor pressure vs.temperature curves for diethyl ether,ethanol,mercury,and water,not necessarily in that order. 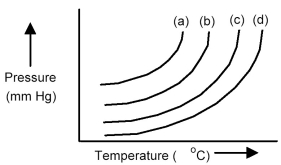
-Based on the relative strengths of the intermolecular forces of attraction of each substance,which is the most likely vapor pressure vs.temperature curve for water?
A) curve (a)
B) curve (b)
C) curve (c)
D) curve (d)
Correct Answer:
Verified
Q38: The highest coordination number for spherical packing
Q39: How many atoms are in one face-centered
Q40: Lead has a radius of 154 pm
Q41: How many Br- ions are around each
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents