Hydrogen peroxide decomposes to water and oxygen according to the reaction below:
2 H2O2(aq) → 2 H2O(l) + O2(g)
In the presence of large excesses of I- ion,the following set of data is obtained.What is the average rate of disappearance of H2O2(aq) in M/s in the first 45.0 seconds of the reaction if 1.00 L of H2O2 reacts at 25°C and 1.00 atm pressure? 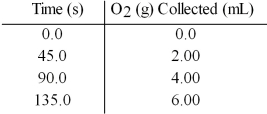
A) 9) 09 × 10-7 M/s
B) 3) 63 × 10-6 M/s
C) 4) 33 × 10-5 M/s
D) 1) 64 × 10-4 M/s
Correct Answer:
Verified
Q1: For a reaction that follows the general
Q4: Chlorine reacts with chloroform according to the
Q6: A concentration-time study of the gas phase
Q7: A concentration-time study of the gas phase
Q17: Which term describes the measure of the
Q19: Methanol can be produced by the following
Q20: The decomposition of ammonia to nitrogen and
Q21: The following set of data was obtained
Q22: The following set of data was obtained
Q24: The reaction between chlorine and nitric oxide
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents