The following set of data was obtained by the method of initial rates for the reaction:
S2O82-(aq) + 3 I-(aq) → 2 SO42-(aq) + I3-(aq)
What is the initial rate when S2O82- is 0.15 M and I- is 0.15 M? 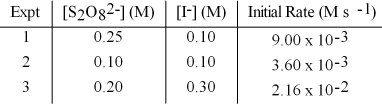
A) 4) 10 × 10-6 M s-1
B) 8) 10 × 10-3 M s-1
C) 1) 22 × 10-2 M s-1
D) 5) 40 × 10-2 M s-1
Correct Answer:
Verified
Q3: What is the overall reaction order for
Q4: Chlorine reacts with chloroform according to the
Q9: Iodide and hypochlorite ion react in aqueous
Q11: Using the method of initial rates for
Q18: The hydrolysis of tert-butyl chloride is given
Q20: The decomposition of ammonia to nitrogen and
Q22: The following set of data was obtained
Q24: The reaction between chlorine and nitric oxide
Q26: The following set of data was obtained
Q28: Given the following hypothetical reaction: 2 E(g)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents