Consider the first-order decomposition of A molecules (shaded spheres) in two vessels of equal volume.What is the half-life of decomposition in vessel (b) relative to the half-life of decomposition in vessel (a) ? 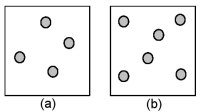
A) half-life in vessel (b) /half-life in vessel (a) = 4:1
B) half-life in vessel (b) /half-life in vessel (a) = 2:1
C) half-life in vessel (b) /half-life in vessel (a) = 3:2
D) half-life in vessel (b) /half-life in vessel (a) = 1:1
Correct Answer:
Verified
Q104: The following pictures represent the progress of
Q106: Consider the first-order reaction A → B
Q125: Consider the first-order decomposition of A molecules
Q126: The following reaction is first order in
Q127: Consider the first-order reaction A → B
Q130: The following reaction is second order in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents