Consider the first-order decomposition of A molecules (shaded spheres) in two vessels of equal volume.How will the rate of decomposition in vessel (a) be affected if the volume of the vessel is decreased by a factor of 2? 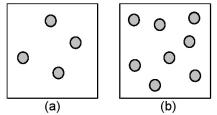
A) decrease by 1/2
B) increase by 2
C) increase by 4
D) stay the same
Correct Answer:
Verified
Q105: Q109: Consider a reaction that occurs by the Q110: Consider the first-order reaction A → B Q132: Consider the first-order reaction A → B Q133: The following reaction is first order in Q134: The following reaction is first order in Q136: The following reaction is second order in Q138: The following reaction is first order in Q139: The following reaction is second order in Q140: The following reaction is second order in![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents