Consider a reaction that occurs by the following one-step mechanism:
A2 + B2 → 2 AB
The potential energy profile for this reaction is shown below. 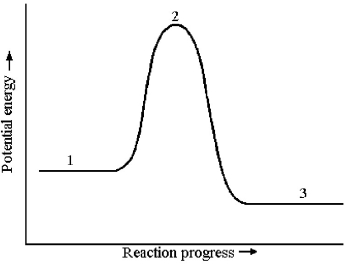
-What is the species present at reaction stage 2?
A) an intermediate
B) a product
C) a reactant
D) a transition state
Correct Answer:
Verified
Q2: The decomposition of dinitrogen pentoxide is described
Q104: The following pictures represent the progress of
Q105: Q106: Consider the first-order reaction A → B Q107: The reaction that occurs in a Breathalyzer,a Q108: Consider a reaction that occurs by the Q110: Consider the first-order reaction A → B Q111: The decomposition of dinitrogen pentoxide is described Q113: The decomposition of dinitrogen pentoxide is described Q114: ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents