Consider a reaction that occurs by the following one-step mechanism:
A2 + B2 → 2 AB
The potential energy profile for this reaction is shown below. 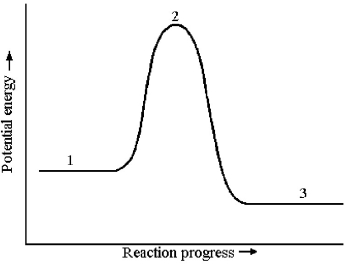
-The energy of reaction,ΔE,is given by the difference in energy between which two reaction stages?
A) reaction stage 2 - reaction stage 1
B) reaction stage 2 - reaction stage 3
C) reaction stage 1 - reaction stage 3
D) reaction stage 3 - reaction stage 1
Correct Answer:
Verified
Q2: The decomposition of dinitrogen pentoxide is described
Q103: The following pictures represent the progress of
Q104: The following pictures represent the progress of
Q105: Q106: Consider the first-order reaction A → B Q107: The reaction that occurs in a Breathalyzer,a Q109: Consider a reaction that occurs by the Q110: Consider the first-order reaction A → B Q111: The decomposition of dinitrogen pentoxide is described Q113: The decomposition of dinitrogen pentoxide is described![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents