The reaction that occurs in a Breathalyzer,a device used to determine the alcohol level in a person's bloodstream,is given below.If the rate of appearance of Cr2(SO4) 3 is 1.64 mol/min at a particular moment,what is the rate of disappearance of C2H6O at that moment? 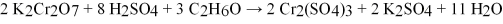
A) 0) 547 mol/min
B) 1) 09 mol/min
C) 2) 46 mol/min
D) 4) 92 mol/min
Correct Answer:
Verified
Q101: Consider a reaction that occurs by the
Q102: Q108: Consider a reaction that occurs by the Q109: Consider a reaction that occurs by the Q117: Q120: Consider a reaction that occurs by the Q144: Which of the following figures represents the Q146: Which of the following figures represents the Q150: The decay curve shown below approximates the Q151: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

