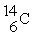
has a decay constant,k = 1.209 × 10-4 yr-1 and a half-life of ________ years.
Correct Answer:
Verified
Q162: The reaction below is second order in
Q168: A reaction has the rate law Rate
Q181: A reaction with an activation energy,Ea =
Q182: The rate-determining step in a reaction is
Q184: Platinum in the catalytic converter of an
Q185: At an elevated temperature the decomposition of
Q187: Hydrochloric acid in the hydrolysis of an
Q194: For the reaction shown below,if the rate
Q195: For the reaction shown below,the rate of
Q196: The aquation of tris(1,10-phenanthroline)iron(II)in acid solution takes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents